Introduction: Why Pharma is Ready for GenAI Agents
The Pharmaceutical and Life Sciences industry operates at a unique and complex intersection of groundbreaking science, massive data volumes, strict regulatory oversight, and global collaboration. Every stage of the value chain—from early molecule discovery to market launch—relies on extensive documentation, rigorous standard operating procedures (SOPs), complex regulatory submissions, and multi-stakeholder reviews.
Despite significant investments in digital and data transformation over the past decade, persistent productivity bottlenecks remain. These challenges are largely driven by fragmented legacy systems, siloed information, and compliance-heavy workflows that still depend on manual intervention. Generative AI (GenAI) introduces a fundamentally new paradigm—shifting the industry from digital automation to cognitive acceleration. In this model, AI agents do more than process data; they can understand context, reason across information, and act within complex business processes.
Pharma’s operating environment is rich in documents, structured data, and deep domain expertise, making it uniquely well-suited for the next generation of intelligent, agent-based AI systems. These GenAI agents can read, reason, summarize, compare, and generate insights at a speed and level of consistency that far exceeds traditional automation or analytics tools.
.png)
From Pilots to Platforms: Embedding GenAI as the New Operating Model
Most pharmaceutical organizations have already begun experimenting with GenAI through targeted pilots. These early initiatives typically include drafting regulatory documents, summarizing clinical trial data, or enabling conversational analytics for internal teams. While such pilots demonstrate clear value, they often remain isolated and fail to scale across the enterprise.
To deliver sustained and measurable impact, GenAI initiatives must evolve from fragmented experiments into a cohesive enterprise platform and operating model—one that embeds intelligence directly into core business workflows rather than treating AI as a standalone capability.
An Enterprise GenAI Platform for Pharma should be built on four foundational pillars:
- Process-aware: Aligned with critical business functions, including R&D, Clinical, Regulatory, Safety, Manufacturing, and Commercial operations.
- Compliance-driven: Integrated with GxP standards, FAIR data principles, and robust data governance to ensure regulatory integrity and patient safety.
- Secure and scalable: Designed with controlled access to enterprise knowledge bases, data lakes, and document repositories to protect intellectual property and sensitive data.
- Agentic by design: Built on modular, reusable GenAI agents that can collaborate across the Pharma value chain to address complex, cross-functional problems.
The Pharma Business Process Map – Where GenAI Creates Value
Below is an overview of Pharma’s value-chain processes and corresponding GenAI agent opportunities:
.png)
Each agent can work independently or as part of a connected network that mirrors the entire pharma end-to-end business flow, linking R&D insights to regulatory submissions, manufacturing documentation, and market operations.
Example: The Power of Knowledge Graph + GenAI Agents
A key innovation area is -RAG (Retrieval-Augmented Generation using Knowledge Graphs) approach. In Pharma, information is dispersed across publications, lab notebooks, safety reports, and regulatory filings.
By integrating knowledge graph intelligence with GenAI reasoning, organizations can:
- Automatically tag and organize scientific and clinical documentation.
- Accelerate literature review and hypothesis generation.
- Enable conversational data exploration (e.g., “Show me trials with adverse reactions similar to compound X”).
Here, Knowledge Graph Agents continuously populate, validate, and enrich the graph using automation, while Analytics Agents leverage it for insights — reducing weeks of research into minutes.
Blueprint for Building an Enterprise GenAI Platform
To operationalize GenAI, Pharma organizations need a systematic build approach—moving from ad-hoc projects to a unified AI for Healthcare platform.
1. Foundation Layer – Data and Governance
- Integrate data from R&D, Clinical, Regulatory, and Commercial systems.
- Ensure governance aligned with HIPAA, GxP, and data lineage standards.
- Build ontology and terminology maps (e.g., SNOMED, MedDRA, MeSH, CTD).
2. AI/ML + GenAI Layer
- Implement multi-modal models for text, image, molecular, and numeric data.
- Use Graph-RAG, vector databases, and LLM fine-tuning for domain-specific needs.
- Maintain an agent orchestration framework to enable collaboration between task-specific agents.
3. Application & Agent Layer
- Develop reusable GenAI agents for each business process area.
- Include guardrails for auditability, human-in-the-loop validation, and explainability.
4. Platform Services
- Integrate with enterprise IAM, workflow engines, document management, and analytics systems.
- Offer APIs and Copilot interfaces for scientists, regulators, and managers.
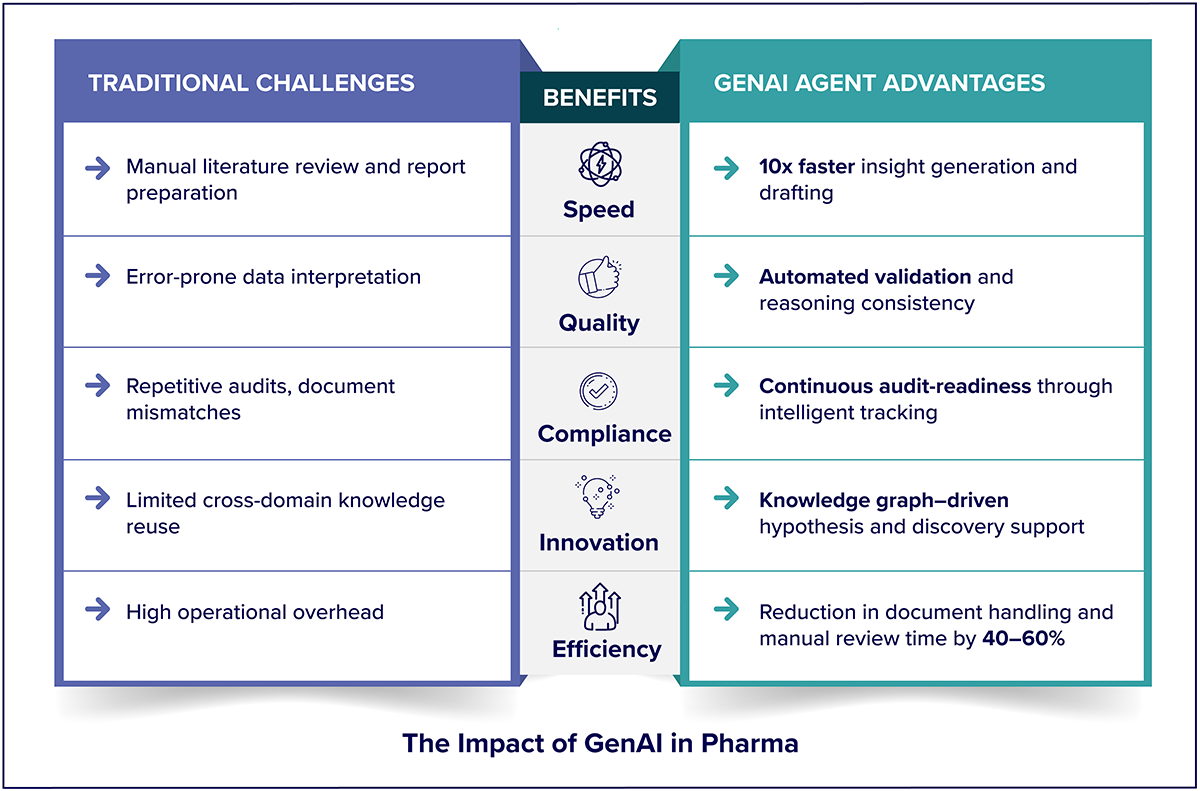
Business Benefits and Measurable Impact
GenAI delivers measurable benefits across the Pharma enterprise:
Example Agent Scenarios
To visualize this in practice, consider these specific agent roles:
- Obligations Advisor Agent – Scans thousands of global vendor and R&D contracts, identifies compliance risks, and summarizes obligations by geography or vendor.
- Clinical Protocol Assistant – Suggests trial designs based on historical protocols, population data, and regulatory precedents.
- Regulatory Document Generator – Automatically assembles submission-ready CTD modules from structured data and templates.
- Pharmacovigilance Case Processor – Extracts and validates adverse event information across case reports and safety narratives.
- Medical Insights Copilot – Synthesizes real-world data, publications, and KOL discussions into actionable medical strategies.
Conclusion: From Digital Pharma to Cognitive Pharma
As GenAI continues to mature, leading Pharma organizations will move beyond isolated automation to Cognitive Operating Models, where AI agents work as trusted partners across science, compliance, and operations.
To reach this stage, organizations should:
- Start with a clear business process map and a well-defined GenAI capability blueprint.
- Build a secure and compliant enterprise GenAI platform with reusable agent frameworks.
- Align technology investments with a domain-led AI for Healthcare vision.
This transformation is not about deploying a chatbot. It is about redesigning how knowledge flows, how decisions are made, and how compliance is maintained in one of the most knowledge-intensive industries in the world.
Building a domain-aligned, secure, and compliant GenAI platform for Pharma and Healthcare is not just a technology task. It requires deep domain knowledge, cloud-native engineering, and strong AI governance.
Relevance Lab brings this combination through its proven work across Cloud, DevOps, Data, and AI Automation. With experience delivering AI-driven platforms in regulated industries, Relevance Lab helps Pharma organizations move from early pilots to scalable, enterprise-ready implementations with confidence.
Key Differentiators
- Deep domain experience in Pharma and Life Sciences: Hands-on experience in clinical data management, regulatory automation, pharmacovigilance, and research workflows.
- AI engineering and GenAI platform expertise: Strong capability in building Graph-RAG architectures, agent-based frameworks, and domain-tuned language models for knowledge-intensive use cases.
- Cloud-native and secure architecture design: Expertise across AWS, Azure, and hybrid environments, with a strong focus on GxP compliance, data privacy, and system monitoring.
- Reusable frameworks and accelerators: The AI Catalyst and Agentic Ops frameworks provide a practical blueprint to speed up AI adoption while keeping costs and governance under control.
- Collaborative co-creation model: Close partnership with customer teams, domain experts, and data scientists to design and build AI solutions that scale and are adopted across the business.
By partnering with Relevance Lab, Pharma organizations can co-create a unified GenAI platform that accelerates discovery and regulatory compliance, while transforming their operating model into one that is data-driven, compliant, and cognitively enabled.

