Introduction
Pharma organizations are increasingly adopting cloud platforms to accelerate drug discovery, modelling, and simulation workloads. However, unlike many other industries, Pharma operates under strict GxP regulations. This means cloud adoption is not just about scalability and performance; it must also support compliance, traceability, and audit readiness.
This blog explores why AWS is well suited for Pharma research workloads, how AWS Research & Engineering Studio (RES) enables self-service research computing, and how Relevance Lab has extended RES to support GxP-regulated research environments in a practical and frictionless way.
Why Pharma Research Workloads Benefit from AWS
Modern drug discovery relies heavily on compute-intensive and data-driven workflows, including:
- Interactive research environments for scientists (RStudio, modelling tools, and exploratory analysis)
- Batch and high-throughput workloads for simulations and parameter sweeps
- PK/PD (Pharmacokinetics/Pharmacodynamics), PBPK (Physiologically Based Pharmacokinetic), and Monte Carlo modelling
- Secure access to sensitive research datasets
Amazon Web Services (AWS) offers Pharma customers a strong foundation for these workloads:
- Elastic compute for bursty modelling and simulation needs
- Secure, isolated environments suitable for regulated research data
- Global-scale infrastructure with enterprise-grade reliability
- A mature compliance ecosystem that supports regulated industries
AWS allows Pharma teams to move from rigid, capacity-constrained on-premises environments to on-demand research computing, accelerating discovery timelines while controlling costs.
The Need for a Self Service Research Portal
AWS offers powerful cloud infrastructure, but scientists should not have to become cloud experts to use it effectively.
Pharma researchers typically want to:
- Launch an approved environment quickly
- Run interactive analyses
- Submit batch jobs for modelling or simulation
- Access data securely
- Focus on science, not infrastructure
This is where AWS Research & Engineering Studio (RES) becomes extremely valuable.
RES provides:
- A self-service portal for launching research workspaces
- Controlled access to compute and storage
- Support for both interactive and batch-oriented workflows
- A consistent experience across research teams
By abstracting cloud complexity, RES enables Pharma organizations to democratize access to compute while retaining centralized governance.
Why Aligning RES with GxP Requirements Is Critical
While RES is a powerful platform, operation within a GxP framework depends on how the platform is configured, validated, and governed by the customer. In regulated Pharma environments:
- Compliance is defined by how a system is deployed, validated, and operated
- Not by any claims of certification or inherent compliance made by a platform
To use RES in GxP-relevant contexts, organizations must ensure:
- Controlled access and role separation
- Reproducible, validated environments
- Traceable operations with audit logs
- Alignment with IQ/OQ/PQ (Installation Qualification, Operational Qualification, and Performance Qualification) models
- Integration with the organization’s Quality Management System (QMS)
Without this alignment, even the most capable cloud platform cannot be confidently used in regulated research.
How Relevance Lab Enhances AWS RES for GxP Use
Relevance Lab has extended AWS RES into a research platform that can be configured, validated, and operated within a GxP framework, by combining cloud-native best practices with Pharma validation expectations.
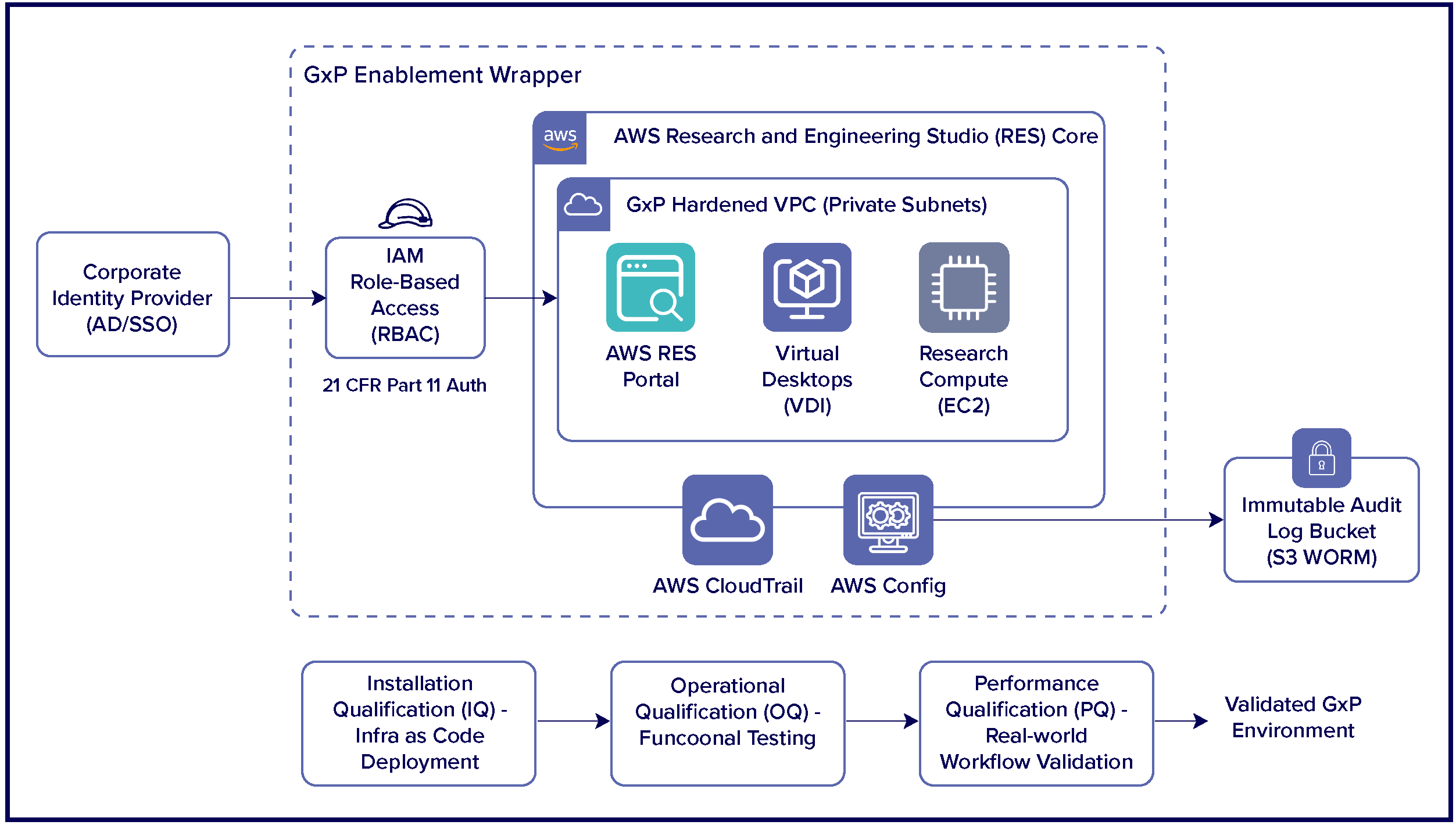
Key enhancements include:
1. GxP-Aligned Architecture
- Private, secure deployment patterns
- Enterprise identity integration
- Encrypted data storage and controlled access
- Centralized, immutable logging
2. Validated Environment Design
- Standardized and version controlled workspace templates
- Validated AMI / image pipelines
- Separation of DEV, VALIDATION, and PROD environments
3. Validation Framework
- Clear mapping of RES capabilities to IQ, OQ, and PQ
- Ready-to-use validation artifacts and templates
- Traceability from intended use to validation evidence
4. Operational Governance
- Defined SOPs for access, change management, and operations
- Audit ready logging and reporting
- Support for periodic reviews and revalidation triggers
These enhancements support the operation of RES in GxP-regulated environments, without compromising the agility that cloud platforms provide.
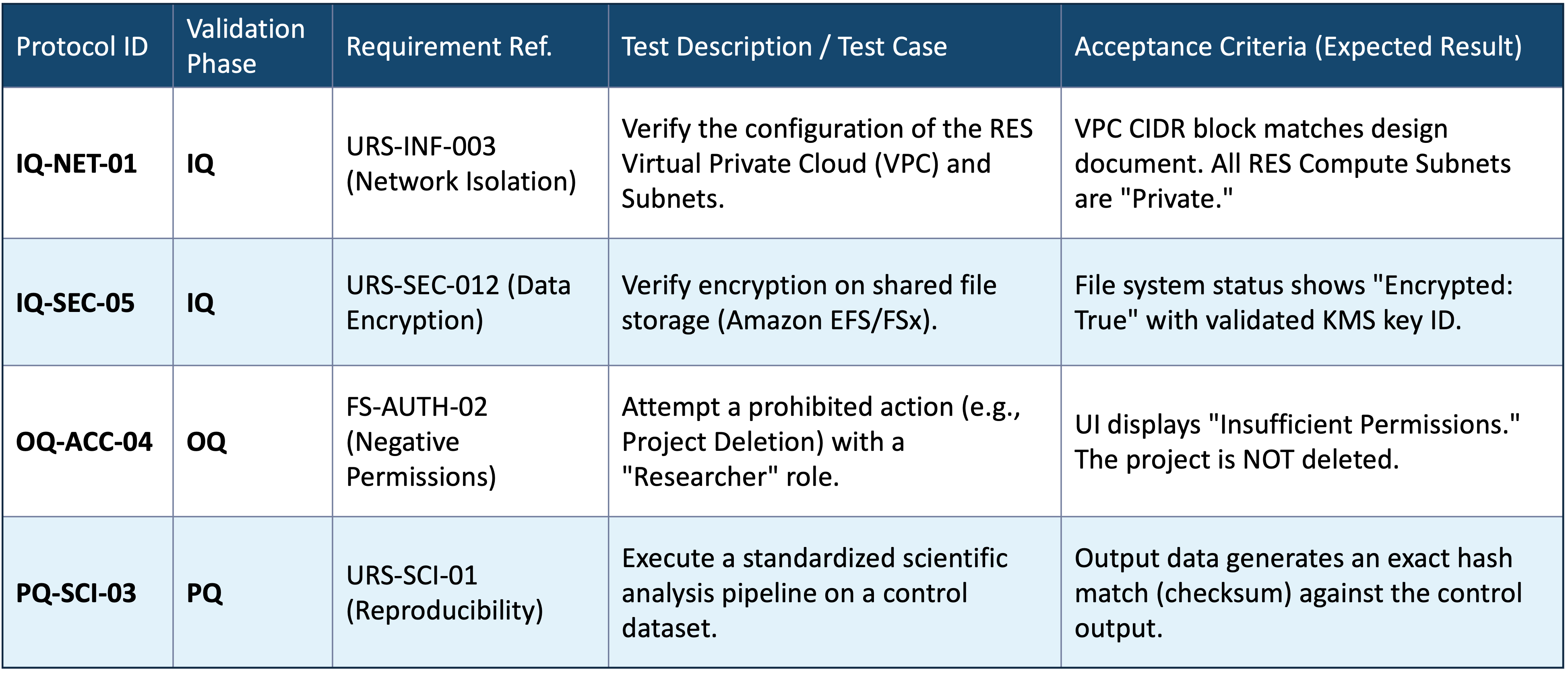
Sample Validation Protocol
A robust validation process requires clear, unambiguous testing. Below is an excerpt from a typical validation protocol we execute for our clients.
A Frictionless Path for New Pharma Customers
One of the biggest barriers to cloud adoption in regulated environments is perceived complexity.
Relevance Lab addresses this by offering:
- A ready-to-adopt RES solution tailored for Pharma
- Clear GxP positioning and validation approach
- A guided implementation model from assessment through steady-state operations
- A live demo environment to demonstrate controlled behaviour in practice
This allows Pharma customers to transition from legacy platforms to AWS-based research environments without disruption, while retaining ownership of compliance.
Conclusion
AWS Research & Engineering Studio provides an excellent foundation for modern Pharma research computing. When combined with a thoughtful GxP strategy and customer-led validation approach, it enables organizations to:
- Empower scientists with self-service access
- Scale interactive and batch workloads efficiently
- Support compliance, traceability, and audit readiness
- Reduce dependence on rigid proprietary platforms
By enhancing RES with GxP enabled architecture, validation frameworks, and operational controls, Relevance Lab helps Pharma customers adopt cloud research platforms with confidence, while retaining ownership of regulatory responsibility.
Partnering for the Future of Science
We understand that time-to-science is critical. Through our RES Enterprise Jump-start Program, we can take an organization from "zero" to a fully deployed, custom-domain, secure RES environment in a few weeks.
Don't let regulation slow down your innovation. Contact Relevance Lab today to modernize your research IT with confidence.
Note: GxP compliance is the responsibility of the regulated organization and depends on system configuration, validation, and operational controls.

